Given the crossover between medical device product development and device and application technology, it only makes sense to adopt an agile approach. Implementing an agile development process provides significant benefits to teams. While some MedTech teams are wary, by taking the time to plan your agile development implementation, your organization can start reaping the benefits.
Implementing an agile development strategy for medical device product development provides numerous benefits for organizations, keeping them competitive in an increasingly saturated market.
Why Use Agile Development Strategies for Medical Device Product Development
Save Time and Money
Due to the inherent iterative nature of agile development strategies, teams can easily make changes early and often in the development process. This helps reduce reworks, which can often be a huge resource drain. In addition, improved collaboration and better processes help to reduce overtime, cut down on costly delays, and shorten release cycles.
Provide Customer Value
The agile method prioritizes adaptation early and often through feedback, testing, and reviews from critical stakeholders. By incorporating customers into this process, teams can take a truly customer-oriented mindset and mold projects around the expectations and needs of customers.
Create High-Quality Products
Agile development strategies provide more opportunities to refine your product, compared to traditional development methods. The flexibility of agile methodology also allows teams to incorporate risk assessments and regulatory requirements all along the way, ultimately developing products of the highest possible quality.
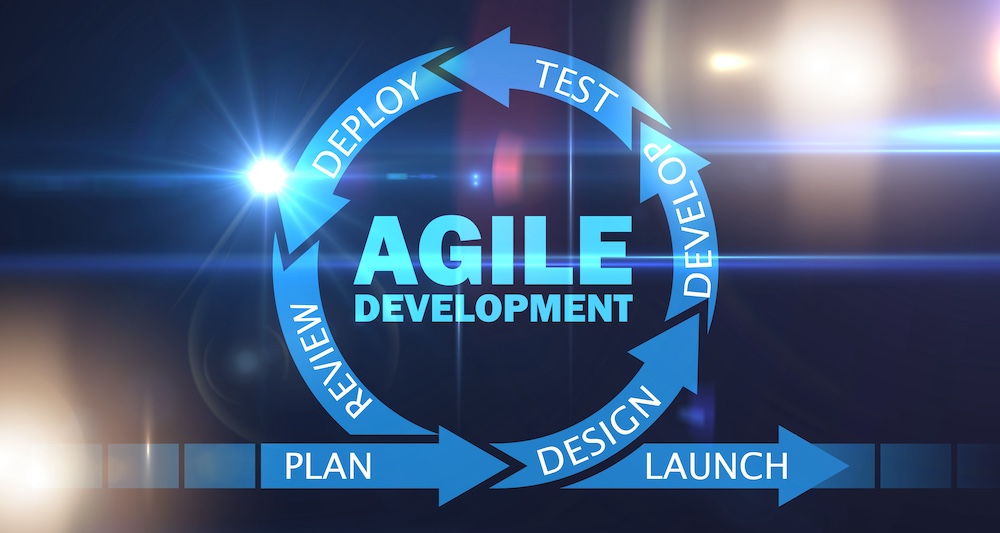
Best Practices for Using Agile for Medical Device Product Development
There’s a lot that goes into the agile journey. If you’re considering implementing an agile approach for your medical device development, here are some best practices to consider:
- Research agile development strategies, specifically which ones are best deployed for medical device product development
- Make sure to get all stakeholders on the same page by establishing the definition of done (DoD), verifying you have proper traceability, and ensuring regulatory compliance
- Build out a core team that will be responsible for the implementation and success tracking of your agile program
- Use the right tools, such as Codebeamer ALM, to support your agile implementation
How to Get Started Implementing Agile for Medical Device Product Development
Making a significant product development transition like this is not easy. Here are a few things you should do to get started:
- Learn and follow the basic principles of agile before you really dig into this endeavor.
- Determine which agile method is right for you (Kanban, LeSS, SAFe®, Scrum, etc.). This will depend on the size of your organization, the types of teams you have, and your company culture.
- Mix and match agile development strategies as you fit the tasks at hand to determine what works best for your team, project, and larger organization.
Changing or implementing an agile development strategy for medical devices requires a thorough understanding of agile frameworks.
Codebeamer: The Ideal Solution for Medical Device Product Development
Codebeamer from PTC makes it easy for established organizations to implement agile development strategies into their operations. It includes pre-built MedTech and agile templates for frameworks like SAFe®, Scrum, or Agile-Waterfall Hybrid. Leveraging these tools allows teams to improve efficiency, quality, and collaboration, all while meeting strict regulatory requirements.
Ready to implement agile into your medical device product development strategies? Or want to learn more about Codebeamer? Contact us.
